In recent years, the landscape of Regulatory affairs has undergone a significant transformation. At the heart of this change is a growing emphasis on patient-centricity, with health authorities (HA) worldwide recognizing the invaluable role patients play in the development and approval of new medicinal products. This shift is not just a trend; it's a fundamental reimagining of how we approach Regulatory submissions and, ultimately, how we serve those who matter most – the patients.
The Problem:
Traditionally, Regulatory submissions focused primarily on meeting technical and scientific requirements, often overlooking the direct impact on patient experiences and outcomes. This approach, while thorough, sometimes fails to capture the nuances of patient needs, preferences, and real-world usage of medicinal products. As a result, there was a disconnect between Regulatory approvals and the actual value delivered to patients.
Patient-Centric Regulatory Approaches:
Health authorities, particularly the FDA and EMA, have taken significant strides in incorporating patient perspectives into their Regulatory frameworks.
This patient-first approach is evident in several key areas:
- Patient-Focused Drug Development (PFDD):
The FDA's PFDD program is a groundbreaking initiative that systematically gathers patient input to inform drug development and Regulatory decision-making. This program includes patient listening sessions, public meetings, and the development of guidance documents to enhance patient engagement throughout the product lifecycle. - Real-World Evidence (RWE):
There's an increasing recognition of the value of real-world data in understanding a product's performance across diverse patient populations. HAs are now more open to considering RWE in Regulatory submissions, providing a more comprehensive view of a product's benefits and risks. - Patient-Reported Outcomes (PROs):
The inclusion of PROs in clinical trials and Regulatory submissions has become increasingly important. These measures directly capture the patient experience, providing valuable insights into symptoms, quality of life, and treatment satisfaction. - Early Engagement and Scientific Advice:
Health authorities are encouraging sponsors to engage early in the development process, often including patient representatives in these discussions. This early dialogue helps align development plans with patient needs and Regulatory expectations. - Patient Involvement in Benefit-Risk Assessments:
Both the FDA and EMA have developed frameworks to incorporate patient perspectives into benefit-risk assessments, ensuring that Regulatory decisions reflect what matters most to patients.
Table: Key Patient-Centric Initiatives by Major Health Authorities
| Health Authority | Initiative | Description |
|---|---|---|
| FDA | Patient-Focused Drug Development (PFDD) | Systematic approach to gathering patient input |
| EMA | Patients and Consumers Working Party (PCWP) | Forum for dialogue with patient and consumer organizations |
| MHRA | Patient and Public Involvement | Strategy to involve patients in Regulatory processes |
| Health Canada | Canadian Drugs and Health Products Engagement Framework | Enhances patient involvement in Regulatory activities |
Role of Regulatory Service Providers:
In this evolving landscape, the role of Regulatory affairs professionals and partners has become more crucial than ever. They serve as the bridge between sponsors, health authorities, and patients, ensuring that Regulatory strategies align with patient-centric approaches.
Key responsibilities include:
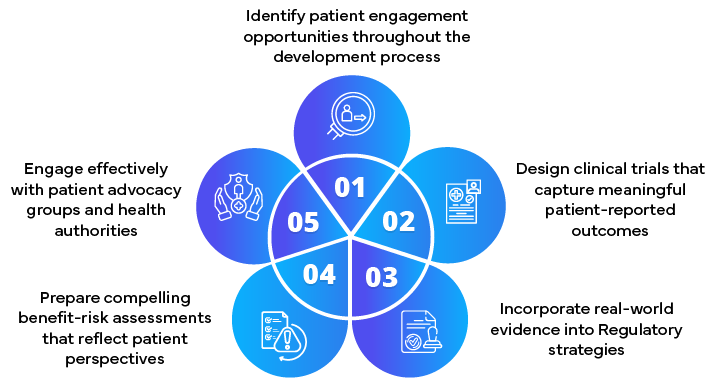
- Developing patient engagement strategies
- Incorporating patient input into Regulatory submissions
- Advising on the collection and presentation of patient-centric data
- Facilitating early engagement with health authorities
- Staying abreast of evolving patient-centric Regulatory requirements
Role of Regulatory Partners in achieving Patient-Centric submissions
Summary:
The shift towards patient-centricity in Regulatory affairs represents a significant opportunity to develop and approve medical products that truly meet patient needs. By embracing this approach, sponsors can not only navigate Regulatory pathways more effectively but also deliver products that make a meaningful difference in patients' lives. As health authorities continue to refine their patient-centric approaches, the expertise of Regulatory service providers or partners becomes invaluable in ensuring that submissions not only meet technical requirements but also resonate with the experiences and priorities of patients.
In this new era of Regulatory affairs, putting patients first isn't just good practice – it's the key to success.