Clinical trials are the cornerstone of medical advancement, paving the way for groundbreaking treatments and therapies. However, these crucial studies are not without their ethical challenges. As we push the boundaries of medical science, researchers, sponsors, and Regulatory bodies find themselves grappling with complex moral dilemmas that demand careful consideration and balanced solutions.
The fundamental ethical challenge in clinical trials stems from the inherent conflict between the need to advance medical knowledge for the greater good and the imperative to protect the rights, safety, and well-being of individual research participants. This tension manifests in various ethical dilemmas throughout the clinical trial process, from study design to participant recruitment, informed consent, and data management.
Key Ethical Dilemmas in Clinical Trials revolve around:
- Informed Consent:
At the core of ethical clinical research lies the principle of informed consent. However, ensuring truly informed consent is often challenging, especially when dealing with vulnerable populations, complex medical concepts, or situations where the capacity to consent may be compromised. - Placebo Use and Standard of Care:
The use of placebos in clinical trials, particularly in areas where effective treatments already exist, raises significant ethical concerns. Balancing the scientific need for placebo-controlled trials with the ethical obligation to provide the best available care to all participants is a persistent challenge. - Risk-Benefit Assessment:
Determining an acceptable level of risk for research participants, especially in early-phase trials or studies involving novel therapies, is a complex ethical consideration that requires careful evaluation of potential benefits against possible harms. - Equity in Participant Selection:
Ensuring a fair selection of research participants while also achieving scientific validity presents ethical challenges. Issues of justice and equitable access to research benefits must be balanced against scientific requirements and logistical constraints. - Post-Trial Access to Treatment:
The ethical obligation to provide continued access to beneficial treatments for participants after a clinical trial concludes, particularly in resource-limited settings, is a growing concern in global clinical research. - Data Privacy and Confidentiality:
With the increasing digitization of clinical trials and the use of big data in research, protecting participant privacy and maintaining data confidentiality have become critical ethical imperatives. - Conflicts of Interest:
Managing potential conflicts of interest among researchers, sponsors, and institutions is crucial to maintaining the integrity of clinical research and public trust in the scientific process.
Table: Ethical Principles in Clinical Trials and Associated Dilemmas
| Ethical Principle | Associated Dilemma |
|---|---|
| Respect for Persons | Ensuring truly informed consent |
| Beneficence | Balancing risks and benefits |
| Justice | Equitable selection of participants |
| Non-maleficence | Use of placebos vs. standard of care |
| Autonomy | Protecting vulnerable populations |
| Transparency | Managing conflicts of interest |
| Confidentiality | Protecting data privacy |
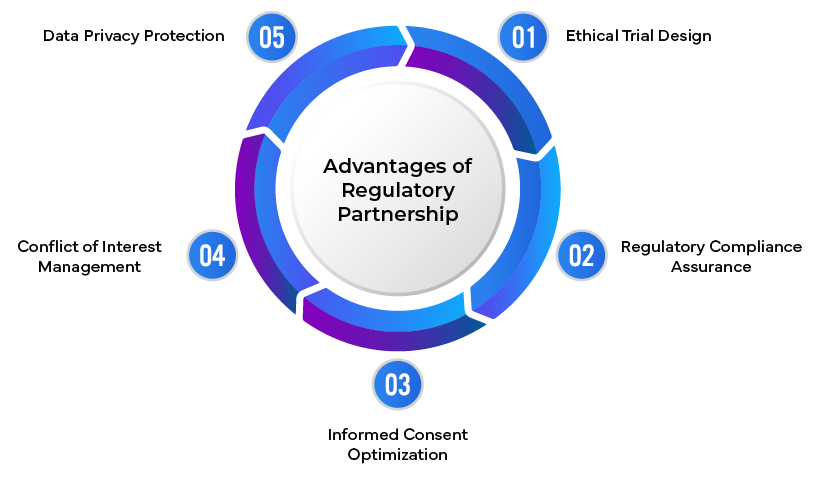
Significance of Regulatory Partnerships:
Navigating these ethical dilemmas in managing clinical trials requires a multifaceted approach, where Regulatory partners play a crucial role.
These partners offer:
- Ethical Guidance: Providing expert advice on ethical considerations in trial design and conduct.
- Regulatory Compliance: Ensuring clinical trials adhere to international ethical guidelines and local regulations.
- Ethics Committee Liaison: Facilitating communication with ethics review boards and helping address their concerns.
- Informed Consent Optimization: Developing clear, comprehensive informed consent processes.
- Conflict Management: Identifying and mitigating potential conflicts of interest.
- Data Protection Strategies: Implementing robust data privacy and confidentiality measures.
- Post-Trial Planning: Developing strategies for post-trial access to treatments.
As we continue to push the boundaries of medical research, addressing these ethical challenges in clinical trials becomes increasingly crucial. By leveraging the expertise of Regulatory partners and adhering to robust ethical frameworks, the clinical research community can navigate these dilemmas more effectively.
This not only ensures the protection of research participants but also maintains public trust in the scientific process, ultimately accelerating the development of life-saving treatments. To further consult on clinical trials and their monitoring, we at Freyr can be your one-stop solution to address all your clinical trials questions.