In the Regulatory landscape of healthcare, combination products represent a frontier of innovation, merging the benefits of drugs, devices, and biologics into single, powerful therapeutic solutions. However, with great innovation comes great responsibility, particularly when it comes to ensuring the safety of our most vulnerable population – children. Focusing on the critical imperatives of child-resistant packaging this blog details how life sciences companies can navigate this complex Regulatory terrain.
The convergence of pharmaceutical and medical device technologies in combination products presents unique challenges in packaging design and Regulatory compliance. Traditional approaches to child-resistant packaging may not suffice for these complex products, leading to potential safety risks and Regulatory hurdles.
Recent recalls of medicinal products from prominent pharma firms due to inadequate child-resistant packaging underscore the importance of this issue and the need for a more nuanced approach to safety and Regulatory compliance.
Considerations to be made:
- Understanding Regulatory frameworks:
Combination products fall under the jurisdiction of multiple Regulatory bodies, primarily the FDA in the United States. The primary mode of action (PMOA) determines which center within the FDA takes the lead in regulation – be it the Center for Drug Evaluation and Research (CDER), the Center for Devices and Radiological Health (CDRH), or the Center for Biologics Evaluation and Research (CBER). For child-resistant packaging, compliance with the Poison Prevention Packaging Act (PPPA) is paramount. - Design Considerations:
Developing child-resistant packaging for combination products requires a delicate balance between safety, usability, and functionality. Innovative packaging designs must not only prevent access by children but also ensure ease of use for adults, particularly seniors or those with disabilities.
This often necessitates novel approaches such as:- Dual-action mechanisms (e.g., push-and-turn caps)
- Visual distraction technologies
- Moisture-activated opening systems
- Perforated blister packs with specific tear patterns
- Testing and Validation:
Rigorous testing is crucial to ensure compliance with child-resistant packaging standards. This typically involves:- Protocol testing with children aged 42-51 months
- Senior-friendly testing to ensure accessibility for adults
- Stability testing to ensure packaging integrity throughout the product's lifecycle
- Regulatory Submissions:
Preparing a comprehensive Regulatory submission for a combination product with child-resistant packaging involves:- Detailed packaging specifications and design rationale
- Results of child-resistant and senior-friendly testing
- Stability data demonstrating packaging effectiveness over time
- Risk analysis and mitigation strategies
Table: Key Regulatory Standards for Child-Resistant Packaging in Combination Products
| Standard | Jurisdiction | Focus |
|---|---|---|
| 16 CFR 1700.20 | United States | Testing protocols for re-closable and non-reclosable packaging |
| ISO 8317 | International | Requirements for re-closable child-resistant packaging |
| EN 14375 | Europe | Non-reclosable packaging for pharmaceutical products |
| BS EN 862:2005 | Europe | Non-reclosable packaging for non-pharmaceutical products |
Role of Regulatory Partners:
Navigating the complex landscape of combination product regulations, especially concerning child-resistant packaging, can be daunting for life sciences companies. This is where Regulatory partners play a crucial role:
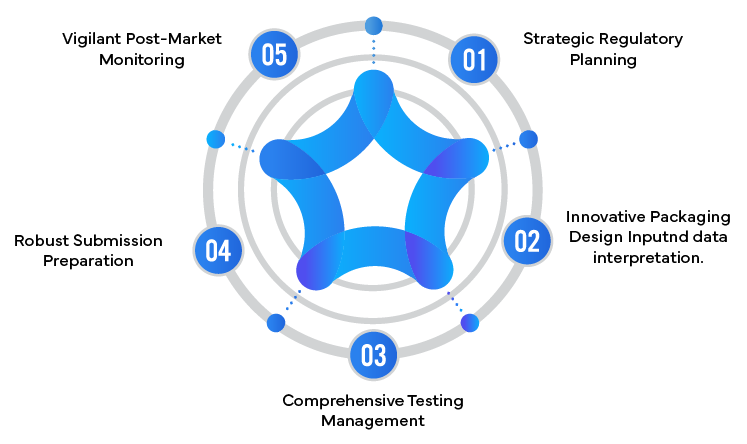
- Regulatory Strategy Development: Crafting a comprehensive strategy that addresses the unique challenges of combination products and child-resistant packaging requirements.
- Design Input: Providing insights on packaging design that meets both Regulatory requirements and user needs.
- Testing Coordination: Managing and interpreting complex testing protocols to ensure compliance with child-resistant standards.
- Submission Preparation: Compiling robust Regulatory submissions that effectively communicate product safety and efficacy.
- Post-Market Surveillance: Implementing systems to monitor combination product performance and safety in real-world settings.
5 Ways Regulatory Partners Enhance Combination Product Safety
Summary:
As combination products continue to push the boundaries of medical innovation, ensuring their safety through effective child-resistant packaging remains paramount. Navigating the complex Regulatory landscape requires a nuanced understanding of both pharmaceutical and medical device regulations, coupled with innovative approaches to packaging design.
By partnering with experienced Regulatory experts, life sciences companies can not only ensure Regulatory compliance but also drive innovation in packaging solutions that protect children while enhancing usability for all patients. In this evolving field, the key to success lies in balancing safety, innovation, and Regulatory acumen – a challenge that, when met, promises to unlock new frontiers in patient care and product design.