In the rapidly evolving landscape of healthcare and life sciences, the intersection of artificial intelligence (AI) and medical writing is ushering in a new era of efficiency, accuracy, and innovation. As the volume and complexity of medical information continue to grow exponentially, AI-driven tools are emerging as powerful allies for medical writers, offering solutions to long-standing challenges and opening new possibilities for creating impactful, patient-centric content. The blog explores the cutting-edge AI innovations in medical writing and their potential to revolutionize healthcare communication.
Medical writers face numerous challenges in today's fast-paced healthcare environment. The sheer volume of scientific literature, the need for rapid dissemination of clinical trial results, and the demand for clear, accurate communication across diverse audiences all contribute to a high-pressure work environment. Traditional methods of research, writing, and quality control are often time-consuming and prone to human error. Additionally, the increasing globalization of healthcare requires content that can be easily adapted for different cultural and linguistic contexts. These challenges call for innovative solutions that can enhance productivity, maintain high standards of accuracy, and improve the overall quality of medical communication.
AI-Driven Innovations in Medical Writing:
- Natural Language Processing (NLP) for Literature Review:
AI-powered NLP tools are revolutionizing the literature review process. These systems can rapidly analyze vast amounts of scientific literature, extracting key information, identifying trends, and summarizing findings. This not only saves time but also ensures a more comprehensive review of available evidence, reducing the risk of overlooking critical information. - Automated Content Generation:
Advanced language models are being adapted for medical writing, capable of generating initial drafts of various documents, from patient education materials to sections of clinical study reports. While human oversight remains crucial, these tools can significantly speed up the writing process and provide a solid foundation for further refinement. - Real-time Writing Assistance:
AI writing assistants offer real-time suggestions for improving clarity, consistency, and adherence to style guidelines. These tools can help maintain a consistent tone across documents, suggest more precise medical terminology, and even flag potential Regulatory compliance issues. - Enhanced Data Visualization:
AI algorithms can analyze complex datasets and automatically generate clear, informative visualizations. This capability is particularly valuable for presenting clinical trial results or epidemiological data in a more accessible format. - Multilingual Adaptation:
AI-powered translation tools, specifically trained in medical terminology, are improving the process of adapting content for global audiences. These systems can maintain the nuanced meaning of medical texts across languages, facilitating more efficient localization of documents. - Predictive Analytics for Clinical Trial Reporting:
AI models can analyze ongoing clinical trial data to predict potential outcomes, helping medical writers prepare more targeted and timely interim reports. This proactive approach can significantly streamline the reporting process for large-scale clinical trials.
Table: Comparison of Traditional vs. AI-Enhanced Medical Writing Processes
| Aspect(s) | Traditional Process | AI-Enhanced Process |
|---|---|---|
| Literature Review | Manual search and analysis | Automated analysis with NLP |
| Initial Draft Creation | A human writer from scratch | AI-generated foundation with human refinement |
| Quality Control | Manual proofreading and fact-checking | AI-assisted error detection and consistency checks |
| Data Visualization | Manual creation of charts and graphs | Automated generation of visualizations |
| Multilingual Adaptation | Human translation and localization | AI-powered translation with human review |
| Regulatory Compliance | Manual checks against guidelines | AI-assisted compliance monitoring |
Role of Clinical Experts:
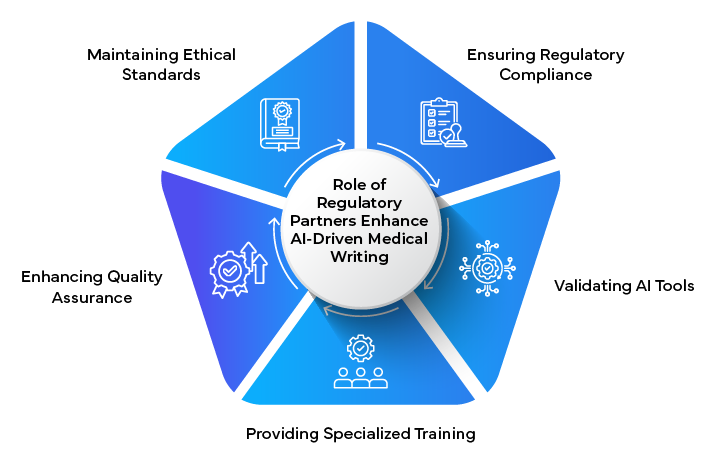
As AI continues to transform medical writing, Regulatory partners particularly their clinical teams play a crucial role in ensuring that these innovations align with industry standards and Regulatory requirements.
Key services provided by Regulatory partners include:
- AI Validation: Assessing and validating AI tools for use in regulated medical writing contexts.
- Compliance Guidance: Providing expertise on how to integrate AI-generated content while maintaining Regulatory compliance.
- Training and Education: Offering programs to help medical writers effectively leverage AI tools while understanding their limitations.
- Quality Assurance: Developing AI-enhanced QA processes that combine machine efficiency with human expertise.
- Ethical Oversight: Ensuring the ethical use of AI in medical writing, particularly concerning data privacy and informed consent.
AI-driven innovations are reshaping the landscape of medical writing, offering unprecedented opportunities to enhance efficiency, accuracy, and the overall quality of healthcare communication. However, the successful integration of these technologies requires a balanced approach that combines the power of AI with human expertise and Regulatory oversight. As we embrace these innovations, the role of Clinical Regulatory partners becomes increasingly vital in navigating the complex intersection of AI, medical writing, and Regulatory compliance.
We at Freyr leverage AI responsibly and strategically and support our clientele to elevate their impact on healthcare by contributing to better patient outcomes and advancing medical science.